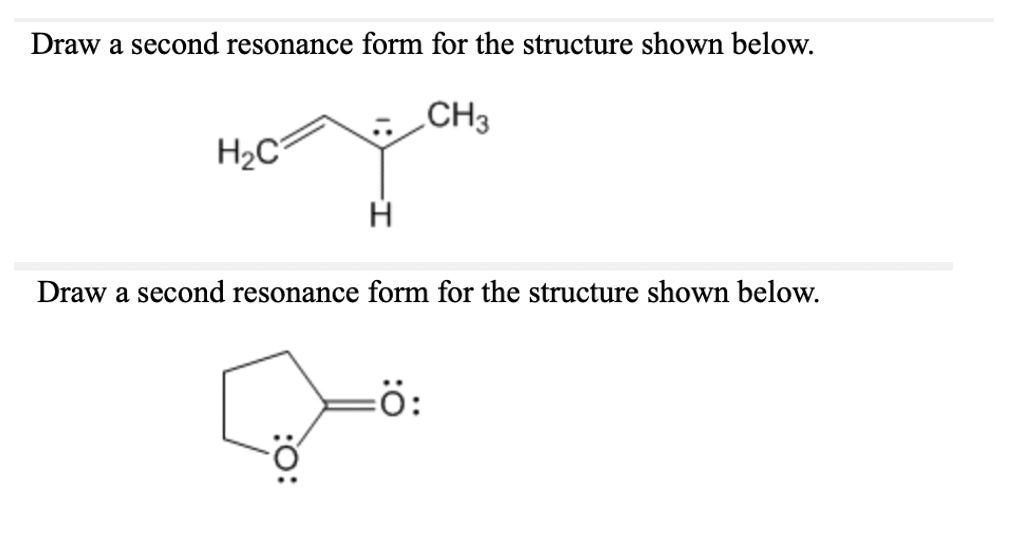
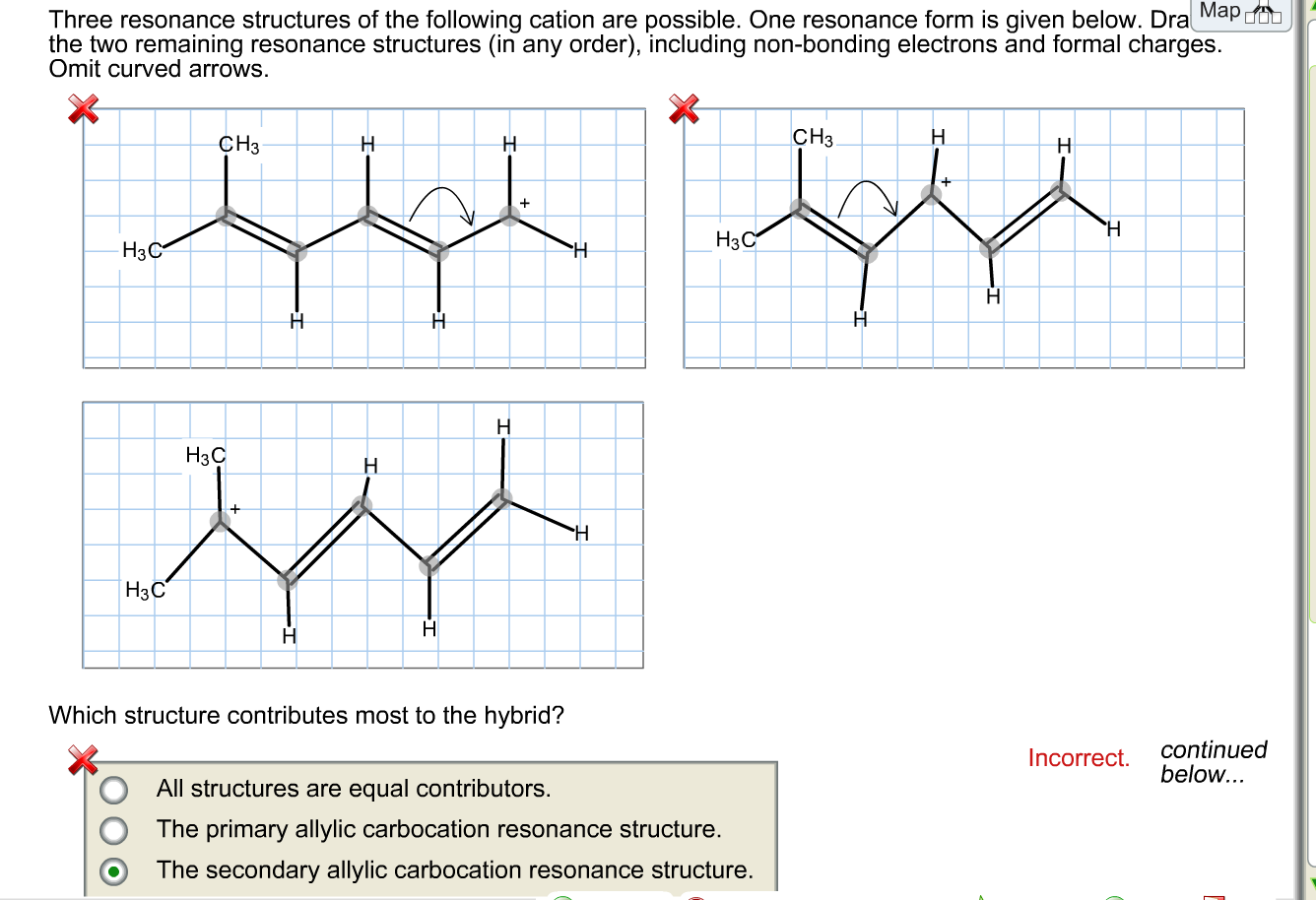
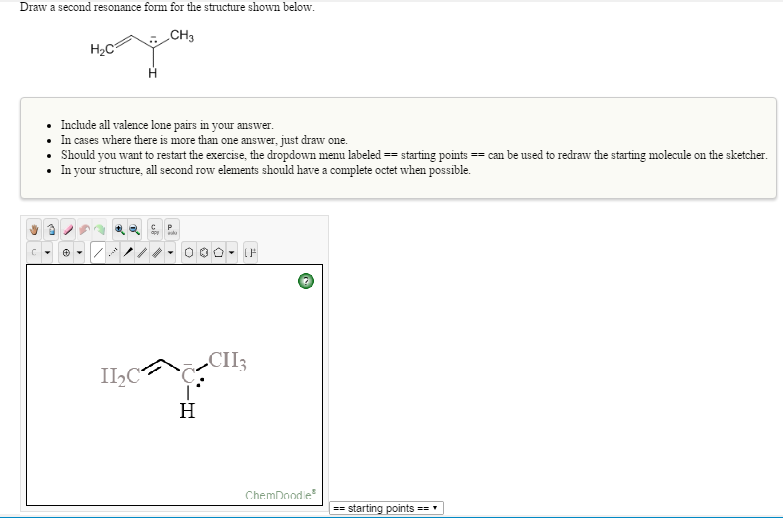
Draw A Second Resonance Form For The Structure Shown Below
Draw A Second Resonance Form For The Structure Shown Below - The objective of this question is to draw the resonance forms for the given molecules. Draw a second resonance form for the structure shown below. The resonance hybrid structure is drawn by the combination of two resonance structures, giving a partial negative charge on both the oxygen. • include all valence lone pairs in. There are 2 steps to solve this one. Each atom should have a complete valence shell and be shown with correct formal charges. A carbocation (carbon with only 6 valence.
A carbocation (carbon with only 6 valence. There are 2 steps to solve this one. The objective of this question is to draw the resonance forms for the given molecules. The resonance hybrid structure is drawn by the combination of two resonance structures, giving a partial negative charge on both the oxygen. • include all valence lone pairs in. Draw a second resonance form for the structure shown below. Each atom should have a complete valence shell and be shown with correct formal charges.
Each atom should have a complete valence shell and be shown with correct formal charges. Draw a second resonance form for the structure shown below. • include all valence lone pairs in. There are 2 steps to solve this one. A carbocation (carbon with only 6 valence. The resonance hybrid structure is drawn by the combination of two resonance structures, giving a partial negative charge on both the oxygen. The objective of this question is to draw the resonance forms for the given molecules.
SOLVED Draw a second resonance form for the structure shown below CH3
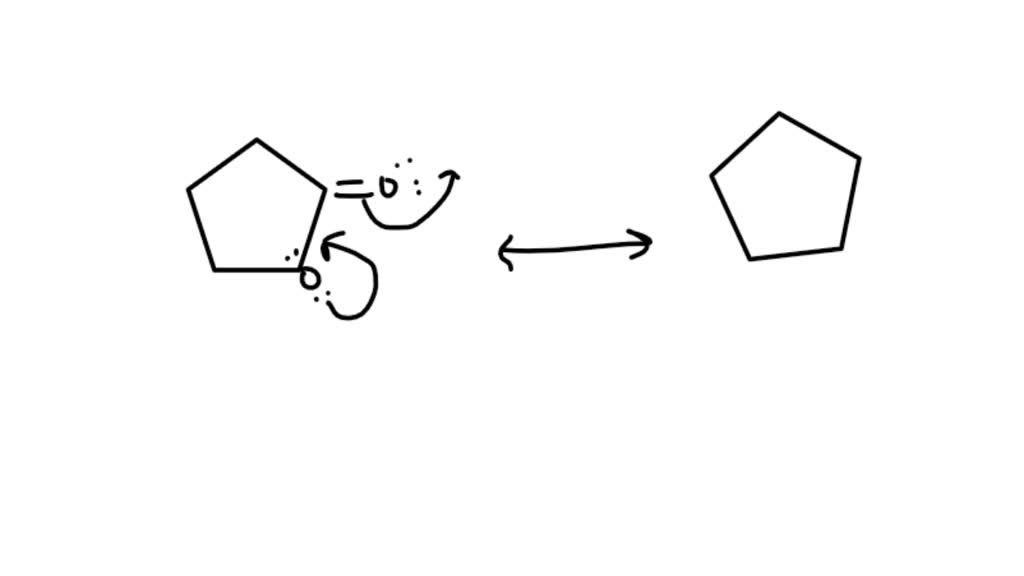
The resonance hybrid structure is drawn by the combination of two resonance structures, giving a partial negative charge on both the oxygen. • include all valence lone pairs in. The objective of this question is to draw the resonance forms for the given molecules. Each atom should have a complete valence shell and be shown with correct formal charges. A.
SOLVED Draw second resonance form for the structure shown below
Each atom should have a complete valence shell and be shown with correct formal charges. The resonance hybrid structure is drawn by the combination of two resonance structures, giving a partial negative charge on both the oxygen. A carbocation (carbon with only 6 valence. Draw a second resonance form for the structure shown below. • include all valence lone pairs.
SOLVED Draw a second resonance form for the structure shown below H3C
The objective of this question is to draw the resonance forms for the given molecules. A carbocation (carbon with only 6 valence. The resonance hybrid structure is drawn by the combination of two resonance structures, giving a partial negative charge on both the oxygen. • include all valence lone pairs in. Each atom should have a complete valence shell and.
[Solved] Draw a second resonance form for the structure shown below. H
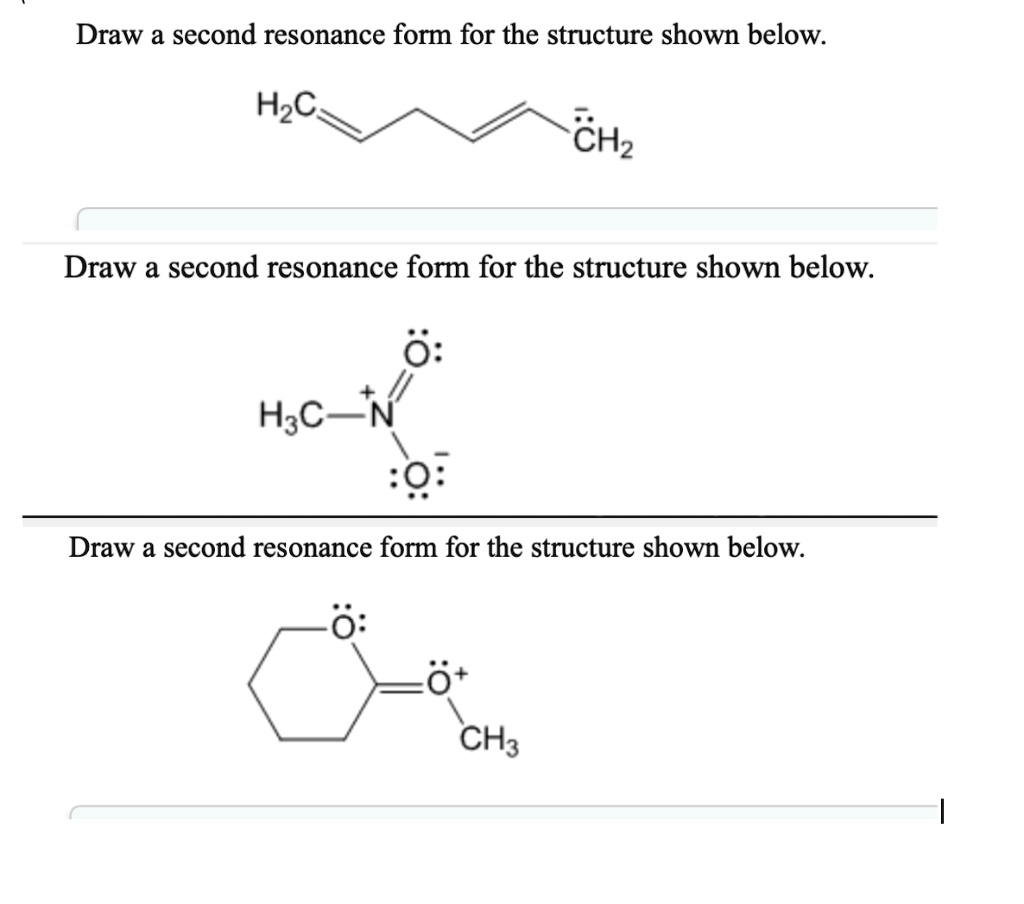
Draw a second resonance form for the structure shown below. • include all valence lone pairs in. There are 2 steps to solve this one. A carbocation (carbon with only 6 valence. The resonance hybrid structure is drawn by the combination of two resonance structures, giving a partial negative charge on both the oxygen.
SOLVED Draw a second resonance form for the structure shown below HzC
The resonance hybrid structure is drawn by the combination of two resonance structures, giving a partial negative charge on both the oxygen. A carbocation (carbon with only 6 valence. Each atom should have a complete valence shell and be shown with correct formal charges. Draw a second resonance form for the structure shown below. The objective of this question is.
[Solved] Draw a second resonance form for the structure shown below. H
• include all valence lone pairs in. The resonance hybrid structure is drawn by the combination of two resonance structures, giving a partial negative charge on both the oxygen. Each atom should have a complete valence shell and be shown with correct formal charges. The objective of this question is to draw the resonance forms for the given molecules. A.
SOLVED Draw second resonance form for the structure shown below
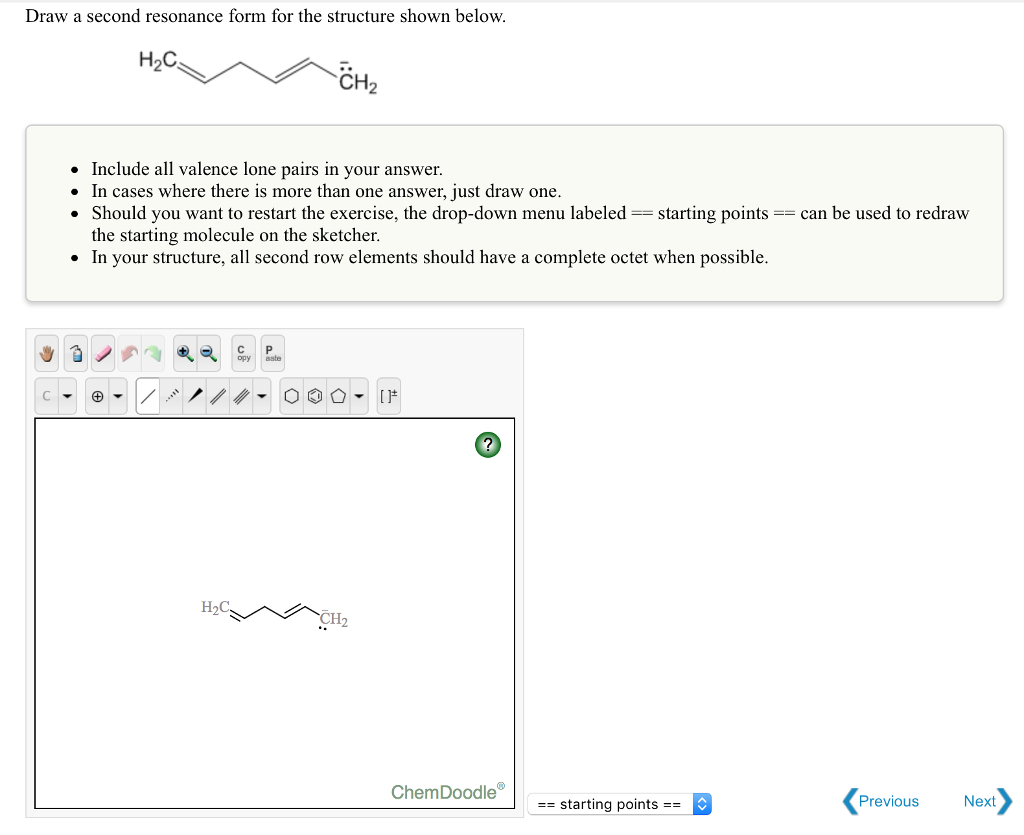
Each atom should have a complete valence shell and be shown with correct formal charges. A carbocation (carbon with only 6 valence. Draw a second resonance form for the structure shown below. There are 2 steps to solve this one. The objective of this question is to draw the resonance forms for the given molecules.
Solved Draw a second resonance form for the structure shown
The resonance hybrid structure is drawn by the combination of two resonance structures, giving a partial negative charge on both the oxygen. • include all valence lone pairs in. The objective of this question is to draw the resonance forms for the given molecules. Each atom should have a complete valence shell and be shown with correct formal charges. Draw.
Draw A Second Resonance Form For The Structure Shown Below
A carbocation (carbon with only 6 valence. The resonance hybrid structure is drawn by the combination of two resonance structures, giving a partial negative charge on both the oxygen. Draw a second resonance form for the structure shown below. Each atom should have a complete valence shell and be shown with correct formal charges. • include all valence lone pairs.
Solved Draw a second resonance form for the structure shown
There are 2 steps to solve this one. Each atom should have a complete valence shell and be shown with correct formal charges. Draw a second resonance form for the structure shown below. The resonance hybrid structure is drawn by the combination of two resonance structures, giving a partial negative charge on both the oxygen. The objective of this question.
A Carbocation (Carbon With Only 6 Valence.
Each atom should have a complete valence shell and be shown with correct formal charges. The resonance hybrid structure is drawn by the combination of two resonance structures, giving a partial negative charge on both the oxygen. • include all valence lone pairs in. There are 2 steps to solve this one.
The Objective Of This Question Is To Draw The Resonance Forms For The Given Molecules.
Draw a second resonance form for the structure shown below.