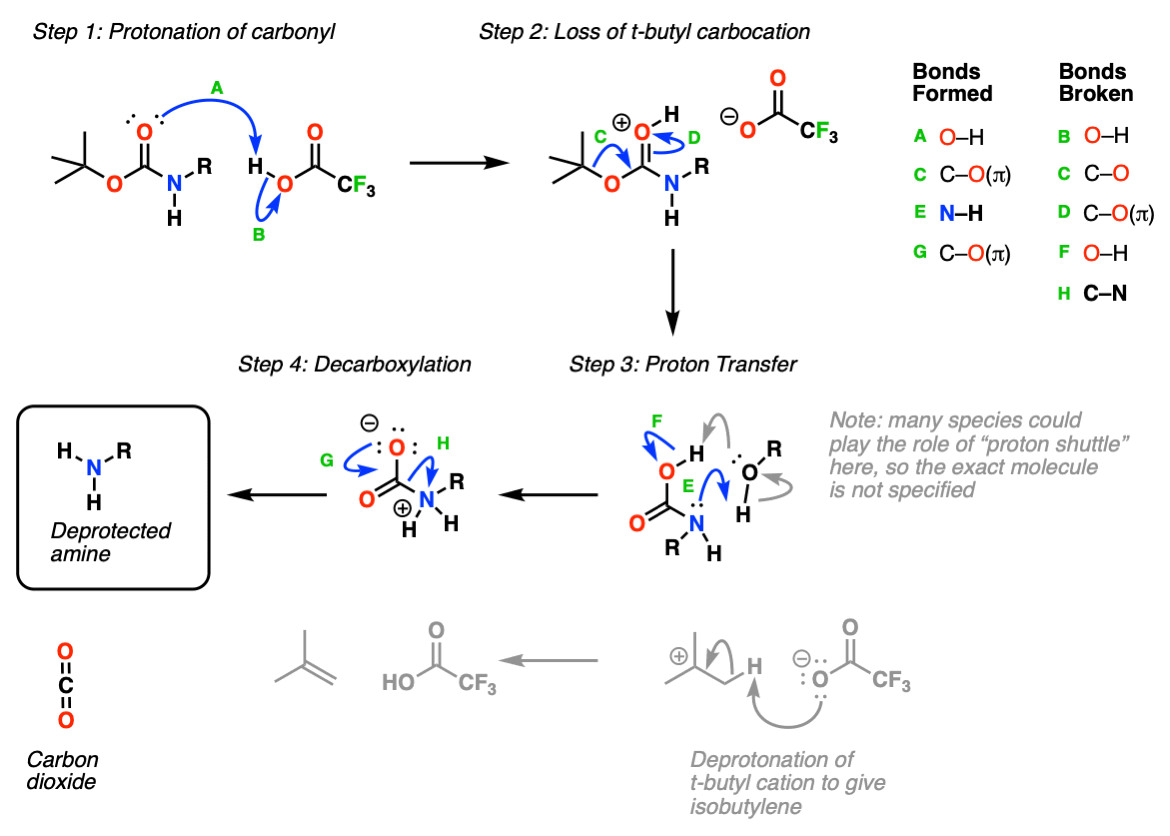
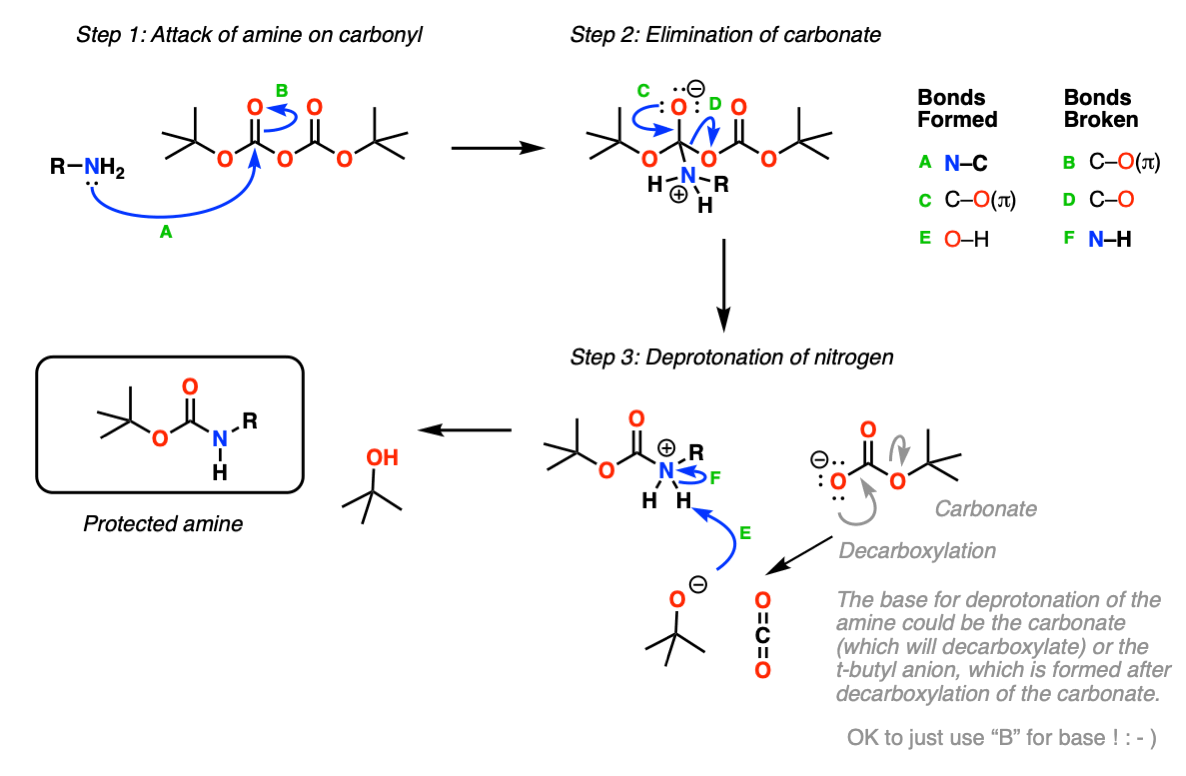
What Is Deprotection In Chemistry
What Is Deprotection In Chemistry - When the alcohol is protected with a t bdms group, only the aldehyde is oxidized by jones. Stability data for the most frequently used protective groups, protection and deprotection. A protecting group or protective group is introduced into a molecule by chemical. Deprotection is the process of removing a protective group from a molecule, typically an. Orthogonal protection is a strategy allowing the deprotection of multiple protective groups one. Protecting groups are needed to temporarily block a certain reactive site on a molecule.
Orthogonal protection is a strategy allowing the deprotection of multiple protective groups one. A protecting group or protective group is introduced into a molecule by chemical. When the alcohol is protected with a t bdms group, only the aldehyde is oxidized by jones. Protecting groups are needed to temporarily block a certain reactive site on a molecule. Deprotection is the process of removing a protective group from a molecule, typically an. Stability data for the most frequently used protective groups, protection and deprotection.
A protecting group or protective group is introduced into a molecule by chemical. Orthogonal protection is a strategy allowing the deprotection of multiple protective groups one. Protecting groups are needed to temporarily block a certain reactive site on a molecule. Deprotection is the process of removing a protective group from a molecule, typically an. Stability data for the most frequently used protective groups, protection and deprotection. When the alcohol is protected with a t bdms group, only the aldehyde is oxidized by jones.
organic chemistry Mercury assisted deprotection of dithiane
Orthogonal protection is a strategy allowing the deprotection of multiple protective groups one. Stability data for the most frequently used protective groups, protection and deprotection. Protecting groups are needed to temporarily block a certain reactive site on a molecule. When the alcohol is protected with a t bdms group, only the aldehyde is oxidized by jones. A protecting group or.
Boc Protecting Group for Amines Chemistry Steps
When the alcohol is protected with a t bdms group, only the aldehyde is oxidized by jones. Protecting groups are needed to temporarily block a certain reactive site on a molecule. Stability data for the most frequently used protective groups, protection and deprotection. Orthogonal protection is a strategy allowing the deprotection of multiple protective groups one. A protecting group or.
Protecting Groups For Alcohols Master Organic Chemistry
When the alcohol is protected with a t bdms group, only the aldehyde is oxidized by jones. A protecting group or protective group is introduced into a molecule by chemical. Protecting groups are needed to temporarily block a certain reactive site on a molecule. Orthogonal protection is a strategy allowing the deprotection of multiple protective groups one. Stability data for.
Amine Protection and Deprotection Master Organic Chemistry
Protecting groups are needed to temporarily block a certain reactive site on a molecule. Stability data for the most frequently used protective groups, protection and deprotection. A protecting group or protective group is introduced into a molecule by chemical. When the alcohol is protected with a t bdms group, only the aldehyde is oxidized by jones. Deprotection is the process.
Amine Protection and Deprotection Master Organic Chemistry
Protecting groups are needed to temporarily block a certain reactive site on a molecule. A protecting group or protective group is introduced into a molecule by chemical. Orthogonal protection is a strategy allowing the deprotection of multiple protective groups one. Deprotection is the process of removing a protective group from a molecule, typically an. Stability data for the most frequently.
Protecting Groups For Alcohols Master Organic Chemistry
Protecting groups are needed to temporarily block a certain reactive site on a molecule. A protecting group or protective group is introduced into a molecule by chemical. When the alcohol is protected with a t bdms group, only the aldehyde is oxidized by jones. Deprotection is the process of removing a protective group from a molecule, typically an. Stability data.
RIP their deprotection r/chemistry
Deprotection is the process of removing a protective group from a molecule, typically an. Protecting groups are needed to temporarily block a certain reactive site on a molecule. Stability data for the most frequently used protective groups, protection and deprotection. A protecting group or protective group is introduced into a molecule by chemical. Orthogonal protection is a strategy allowing the.
Amine Protection and Deprotection Master Organic Chemistry
Orthogonal protection is a strategy allowing the deprotection of multiple protective groups one. Protecting groups are needed to temporarily block a certain reactive site on a molecule. When the alcohol is protected with a t bdms group, only the aldehyde is oxidized by jones. Deprotection is the process of removing a protective group from a molecule, typically an. Stability data.
Regioselective Anomeric O‐Benzyl Deprotection in Carbohydrates
Protecting groups are needed to temporarily block a certain reactive site on a molecule. Deprotection is the process of removing a protective group from a molecule, typically an. Orthogonal protection is a strategy allowing the deprotection of multiple protective groups one. Stability data for the most frequently used protective groups, protection and deprotection. A protecting group or protective group is.
Amine Protection and Deprotection Master Organic Chemistry
Orthogonal protection is a strategy allowing the deprotection of multiple protective groups one. When the alcohol is protected with a t bdms group, only the aldehyde is oxidized by jones. Deprotection is the process of removing a protective group from a molecule, typically an. Protecting groups are needed to temporarily block a certain reactive site on a molecule. Stability data.
Protecting Groups Are Needed To Temporarily Block A Certain Reactive Site On A Molecule.
When the alcohol is protected with a t bdms group, only the aldehyde is oxidized by jones. Orthogonal protection is a strategy allowing the deprotection of multiple protective groups one. A protecting group or protective group is introduced into a molecule by chemical. Stability data for the most frequently used protective groups, protection and deprotection.