Which Group Tends To Form 1 Ions
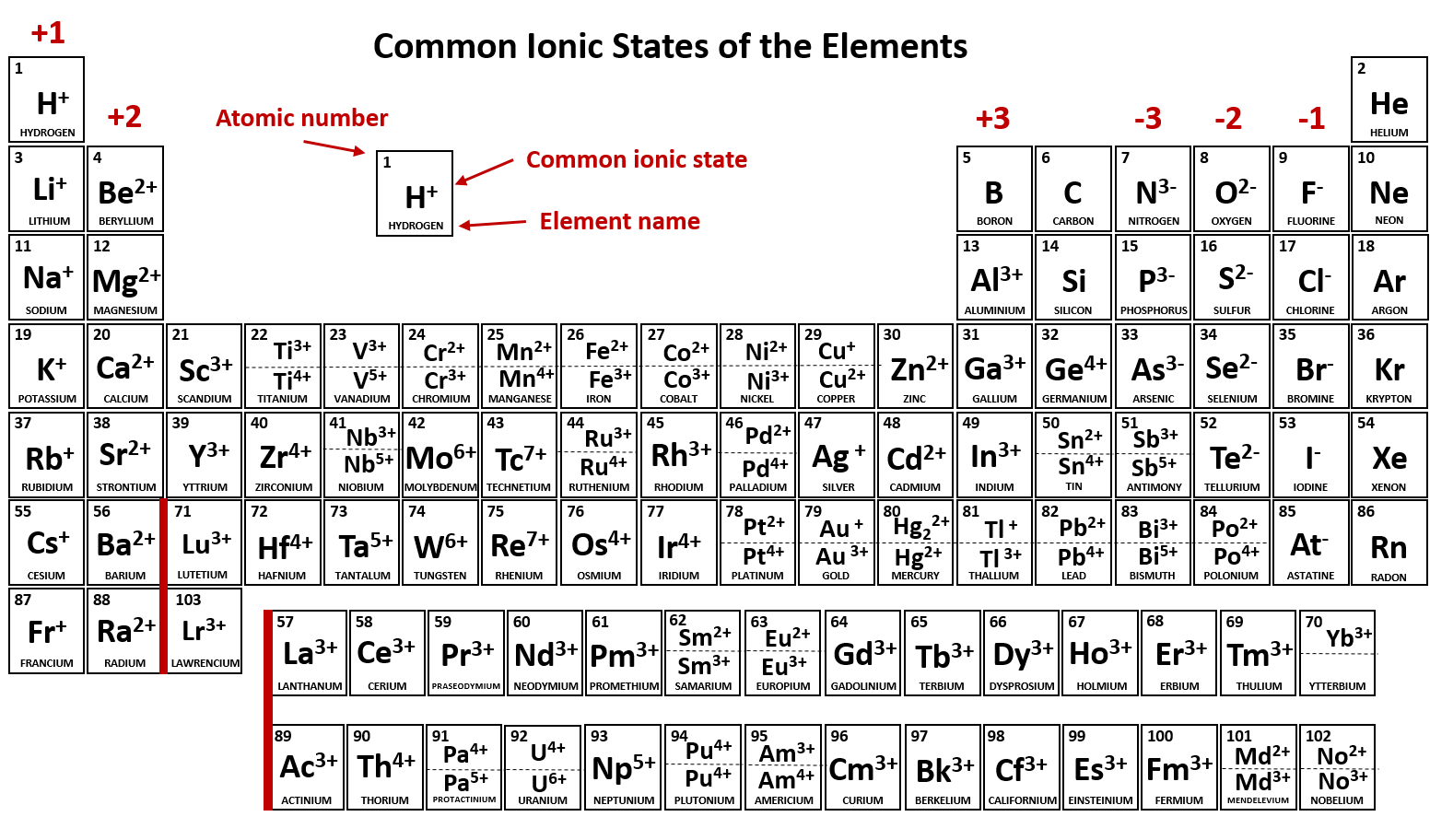
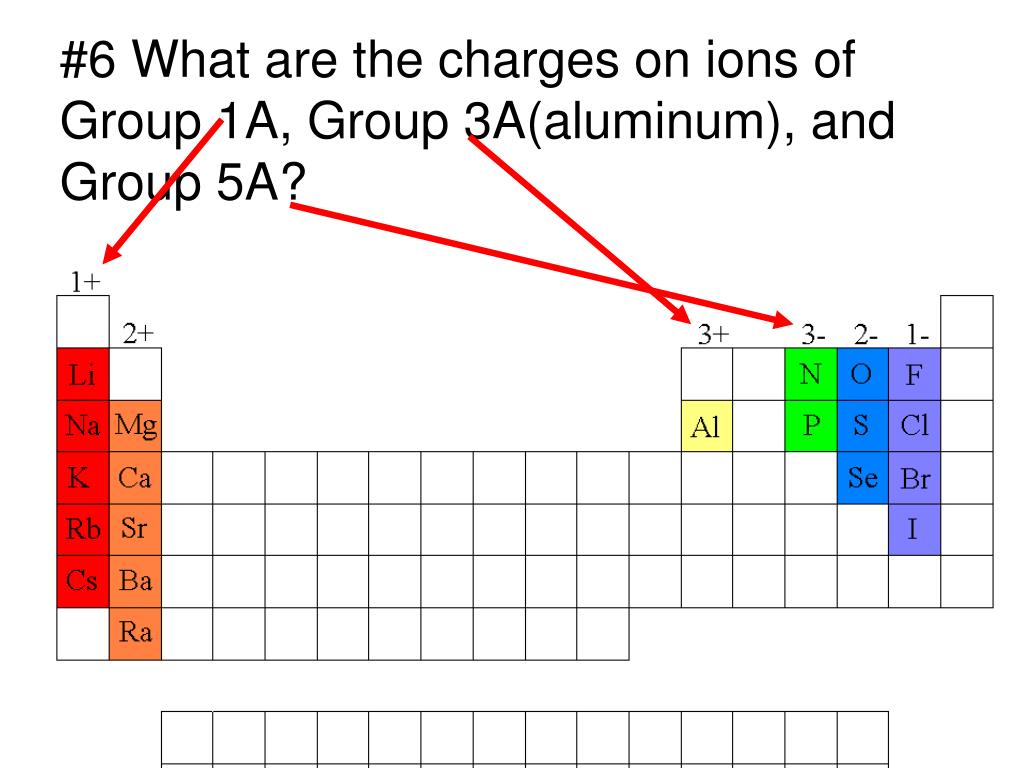
Which Group Tends To Form 1 Ions - The elements in group 1 of the periodic table (alkali metals) tend to form +1 ions. Group 1 metals, the alkali metals, have the 1 valence electron and thus form $$ m ^ {+}$$. The group that tends to form 1+ ions is group 1, which consists of alkali metals such. The charge on a ion indicates the no. Study with quizlet and memorize flashcards containing terms like which group tends to. Group 1 metals, the alkali metals, have the 1 valence electron, and thus form m +. Which periodic group forms +1 ; Which group tends to not form ions or react?
The elements in group 1 of the periodic table (alkali metals) tend to form +1 ions. Study with quizlet and memorize flashcards containing terms like which group tends to. Group 1 metals, the alkali metals, have the 1 valence electron, and thus form m +. The group that tends to form 1+ ions is group 1, which consists of alkali metals such. The charge on a ion indicates the no. Which group tends to not form ions or react? Group 1 metals, the alkali metals, have the 1 valence electron and thus form $$ m ^ {+}$$. Which periodic group forms +1 ;
The charge on a ion indicates the no. Study with quizlet and memorize flashcards containing terms like which group tends to. Which periodic group forms +1 ; Group 1 metals, the alkali metals, have the 1 valence electron, and thus form m +. The group that tends to form 1+ ions is group 1, which consists of alkali metals such. Group 1 metals, the alkali metals, have the 1 valence electron and thus form $$ m ^ {+}$$. The elements in group 1 of the periodic table (alkali metals) tend to form +1 ions. Which group tends to not form ions or react?
Charge Of Ions Calculator
The charge on a ion indicates the no. Group 1 metals, the alkali metals, have the 1 valence electron, and thus form m +. The group that tends to form 1+ ions is group 1, which consists of alkali metals such. The elements in group 1 of the periodic table (alkali metals) tend to form +1 ions. Group 1 metals,.
Periodic Table Which Groups Of Elements Tend To Form Positive Ions
Which group tends to not form ions or react? Group 1 metals, the alkali metals, have the 1 valence electron, and thus form m +. Group 1 metals, the alkali metals, have the 1 valence electron and thus form $$ m ^ {+}$$. The charge on a ion indicates the no. Study with quizlet and memorize flashcards containing terms like.
PPT 1 Name the ions formed by these elements and classify them as
Group 1 metals, the alkali metals, have the 1 valence electron and thus form $$ m ^ {+}$$. Group 1 metals, the alkali metals, have the 1 valence electron, and thus form m +. Which group tends to not form ions or react? The elements in group 1 of the periodic table (alkali metals) tend to form +1 ions. The.
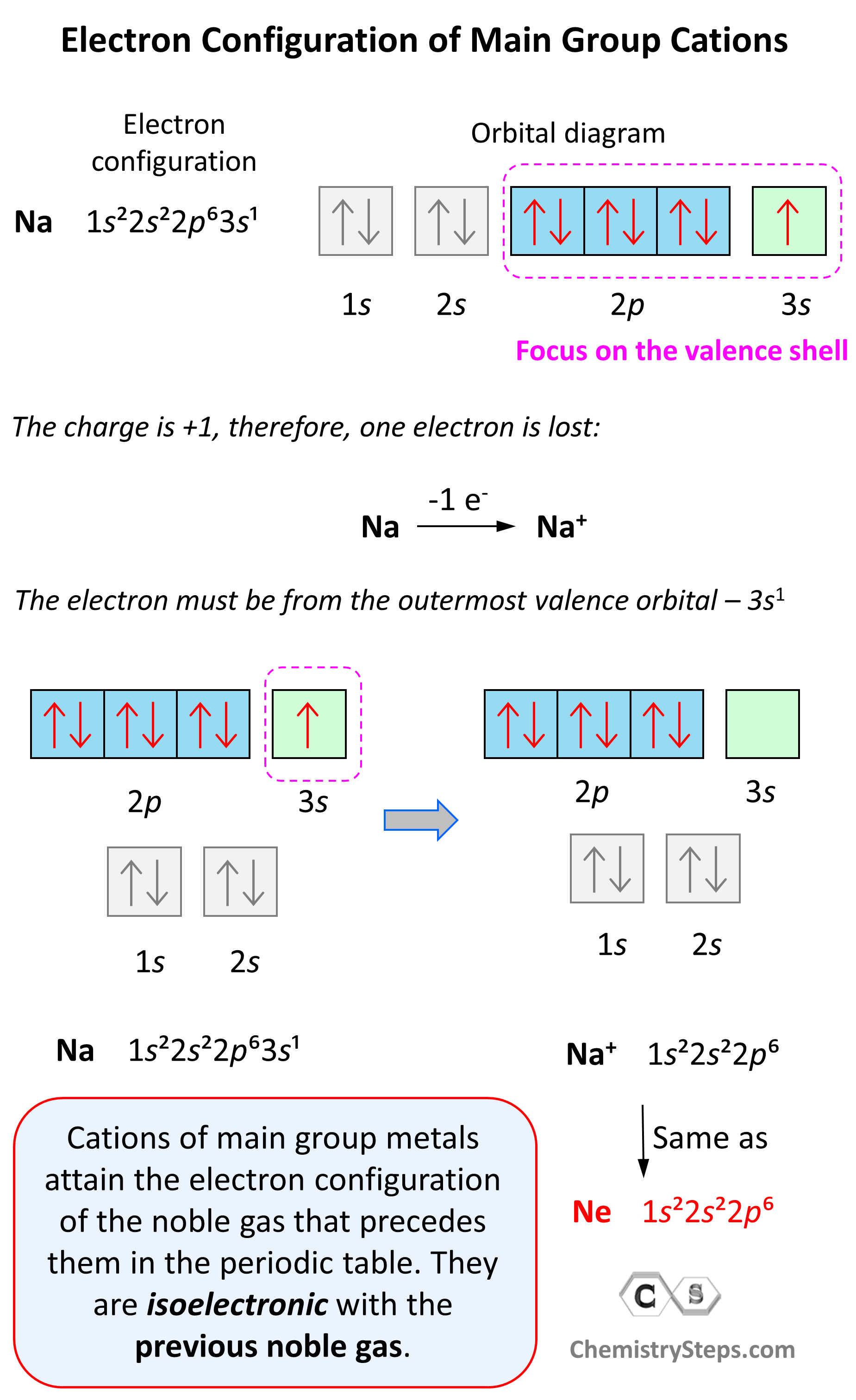
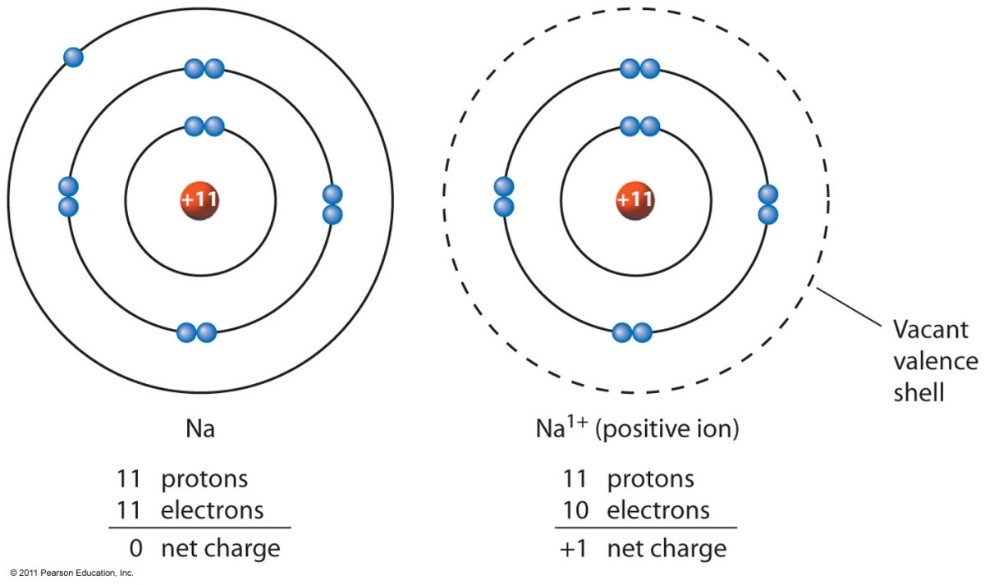
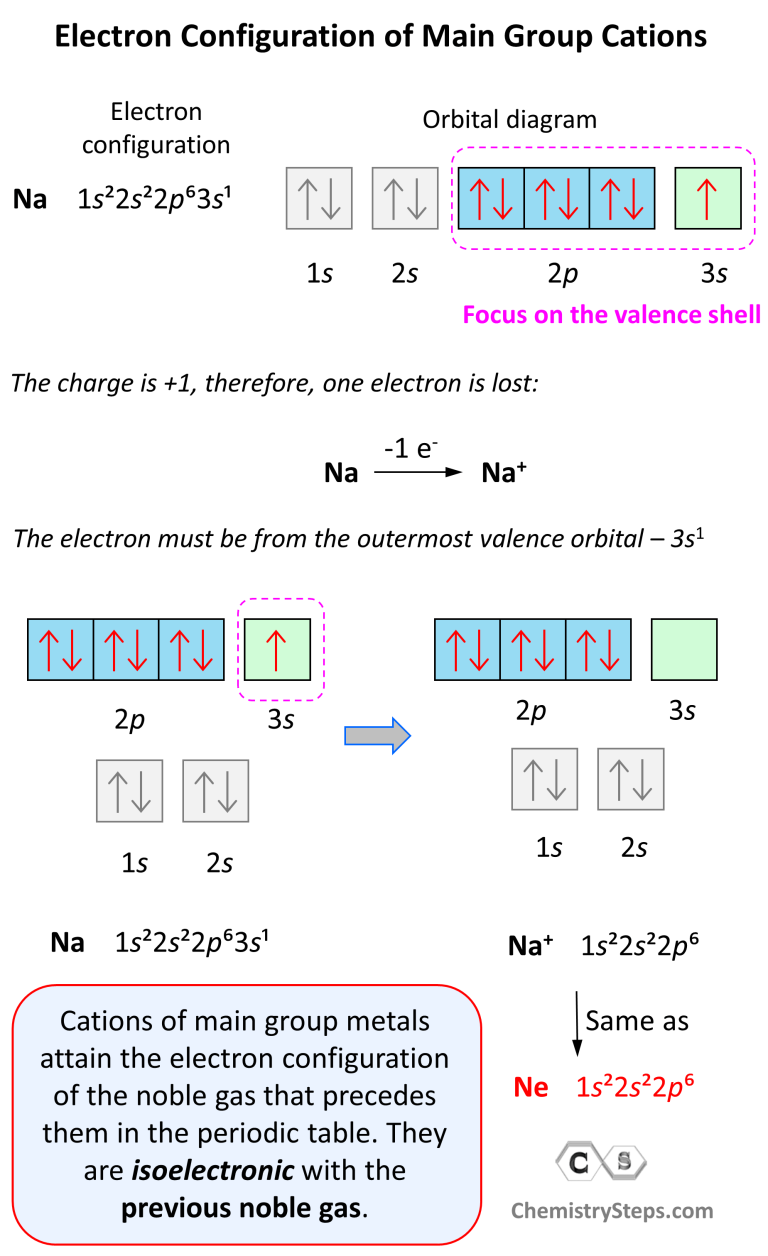
Electron Configurations of Ions Chemistry Steps
The group that tends to form 1+ ions is group 1, which consists of alkali metals such. Group 1 metals, the alkali metals, have the 1 valence electron, and thus form m +. The elements in group 1 of the periodic table (alkali metals) tend to form +1 ions. Study with quizlet and memorize flashcards containing terms like which group.
Naming Simple Ionic Compounds Pathways to Chemistry
Group 1 metals, the alkali metals, have the 1 valence electron and thus form $$ m ^ {+}$$. Study with quizlet and memorize flashcards containing terms like which group tends to. The group that tends to form 1+ ions is group 1, which consists of alkali metals such. Which periodic group forms +1 ; The charge on a ion indicates.
Do Metals Form Positive Or Negative Ions
Group 1 metals, the alkali metals, have the 1 valence electron and thus form $$ m ^ {+}$$. Group 1 metals, the alkali metals, have the 1 valence electron, and thus form m +. The charge on a ion indicates the no. The elements in group 1 of the periodic table (alkali metals) tend to form +1 ions. Which group.
metals tend to form what kind of ions Lombardi Bothe1936
The charge on a ion indicates the no. Which group tends to not form ions or react? Which periodic group forms +1 ; Group 1 metals, the alkali metals, have the 1 valence electron and thus form $$ m ^ {+}$$. Group 1 metals, the alkali metals, have the 1 valence electron, and thus form m +.
Electron Configurations of Ions Chemistry Steps
Which periodic group forms +1 ; Which group tends to not form ions or react? Group 1 metals, the alkali metals, have the 1 valence electron and thus form $$ m ^ {+}$$. Group 1 metals, the alkali metals, have the 1 valence electron, and thus form m +. The elements in group 1 of the periodic table (alkali metals).
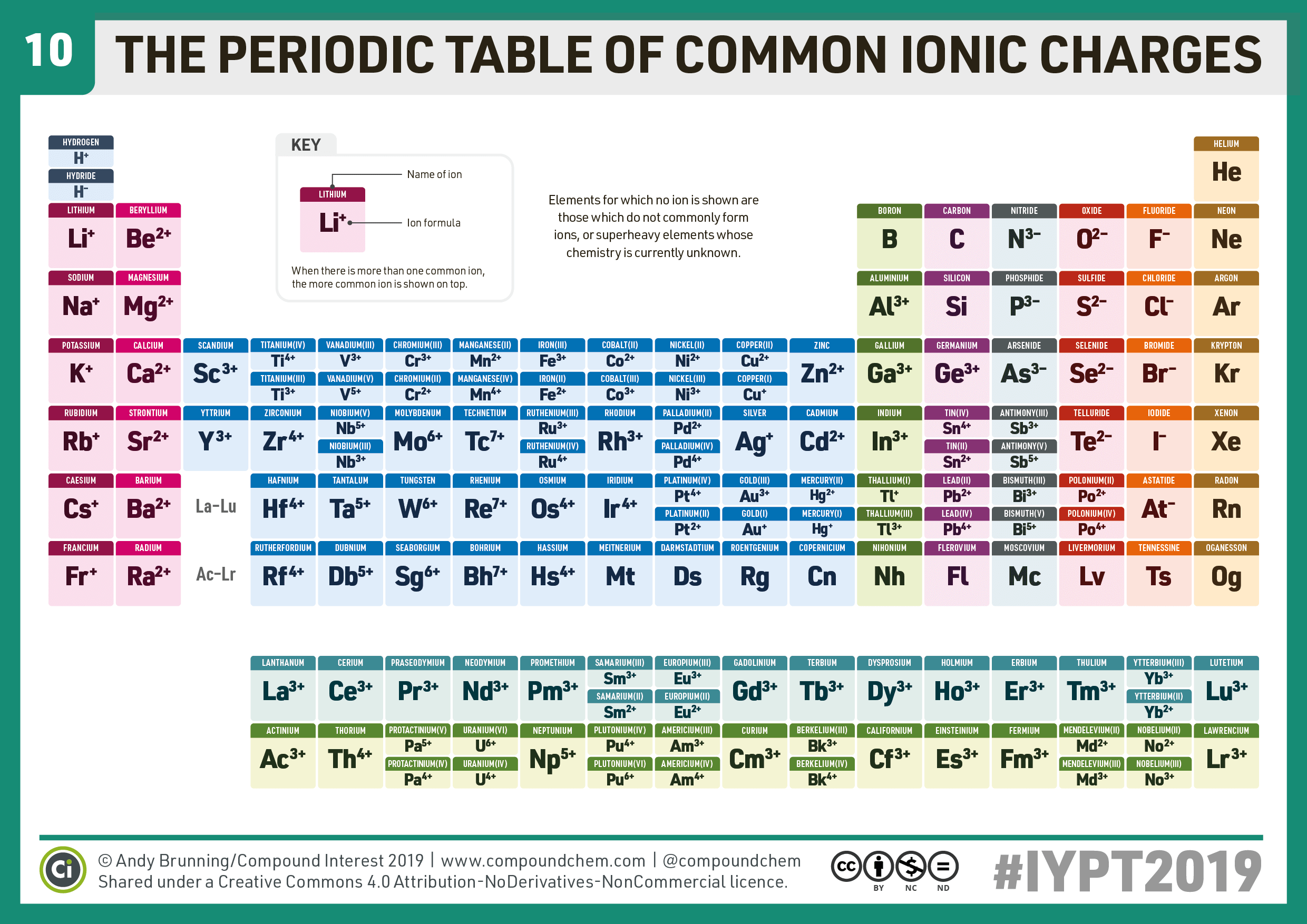
Compound Interest 10 Periodic Table of Common Ions
The group that tends to form 1+ ions is group 1, which consists of alkali metals such. Group 1 metals, the alkali metals, have the 1 valence electron and thus form $$ m ^ {+}$$. Which periodic group forms +1 ; The charge on a ion indicates the no. Which group tends to not form ions or react?
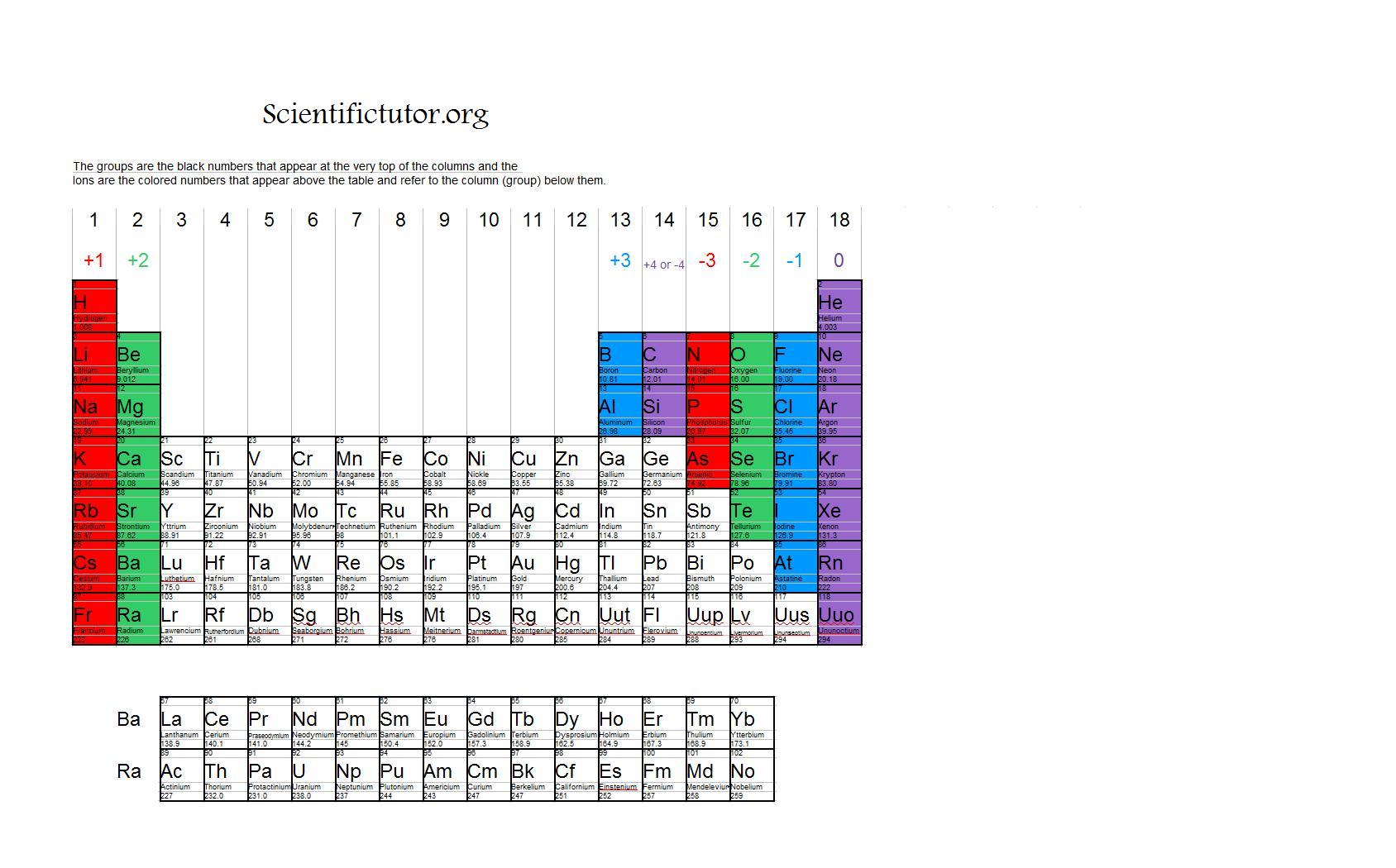
Chem Ions Scientific Tutor
The elements in group 1 of the periodic table (alkali metals) tend to form +1 ions. Group 1 metals, the alkali metals, have the 1 valence electron, and thus form m +. The charge on a ion indicates the no. Which group tends to not form ions or react? The group that tends to form 1+ ions is group 1,.
The Group That Tends To Form 1+ Ions Is Group 1, Which Consists Of Alkali Metals Such.
The charge on a ion indicates the no. The elements in group 1 of the periodic table (alkali metals) tend to form +1 ions. Group 1 metals, the alkali metals, have the 1 valence electron and thus form $$ m ^ {+}$$. Group 1 metals, the alkali metals, have the 1 valence electron, and thus form m +.
Study With Quizlet And Memorize Flashcards Containing Terms Like Which Group Tends To.
Which group tends to not form ions or react? Which periodic group forms +1 ;